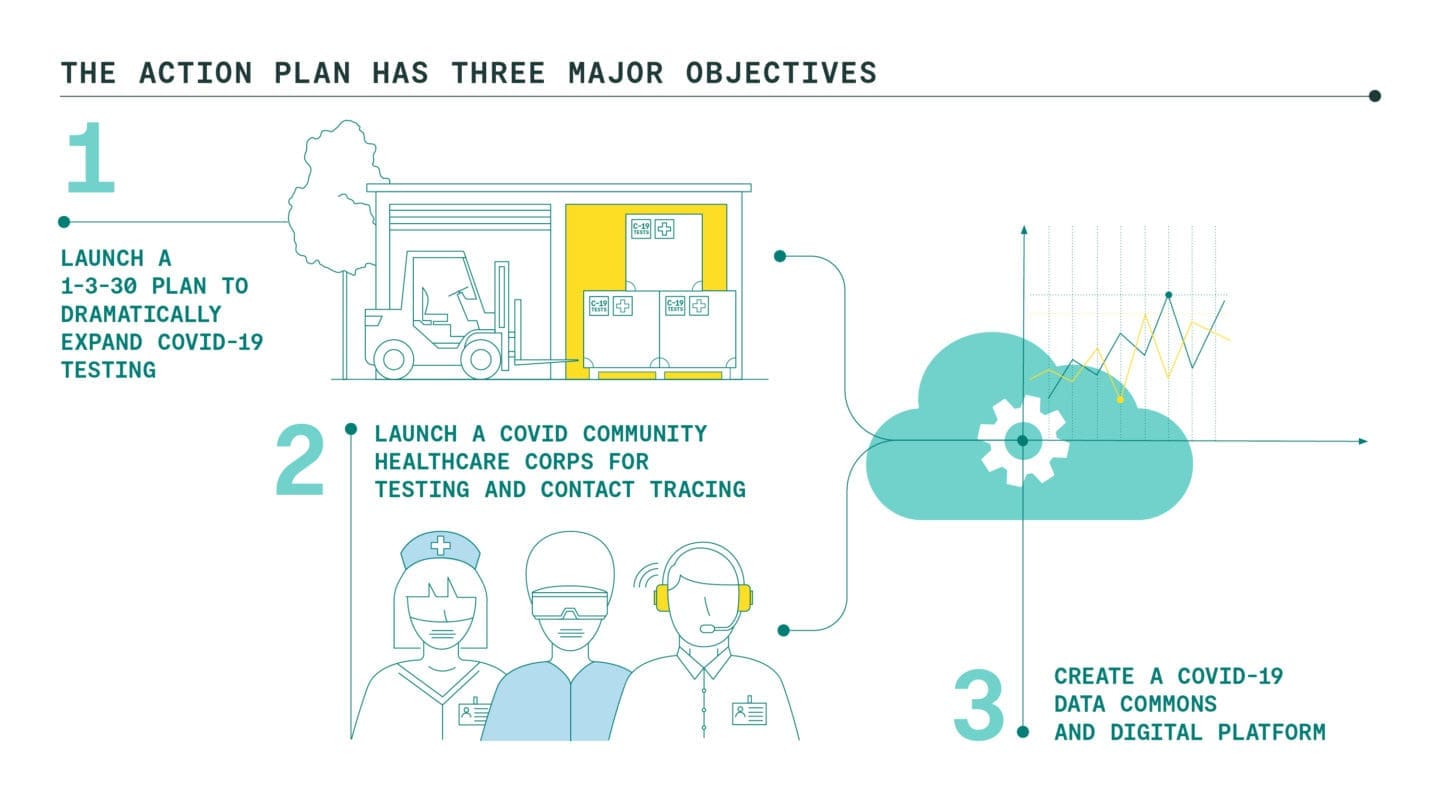
Launch a 1-3-30 Plan to Dramatically Expand Covid-19 Testing
We are proposing our nation come together around the bold, ambitious, but achievable goal of rapidly expanding testing capacity to 30 million tests per week over the next six months. This 1-3-30 Plan would be achieved by: (1) creating an Emergency Network for Covid-19 Testing to coordinate and underwrite the testing market, (2) launching an eight-week National Testing Laboratory Optimization Initiative to increase output to 3 million tests per week from the current one million, and (3) investing in a Testing Technology Accelerator to further grow U.S. testing capacity from 3 million to 30 million tests per week.
The steady increase in U.S. testing that began in late February has now plateaued. During the first two weeks of April, the number of tests per day averaged 143,000 (~ 1 million tests per week) with no appreciable upward trend.[i] As of April 18, 2020, the U.S. had completed 3,698,534 tests of which 722,182 were positive (19.50%)
This undoubtedly reflects just the tip of the COVID-19 pandemic in the U.S. Current barriers to rapid increases in American test production, supply, distribution and administration include uncertainty over financing and payment; lack of coordination of local, state, and national purchases; uneven distribution of test kits; severe shortages of reagents; regulatory barriers; and a severe lack of staffing.
The 1-3-30 Plan aims to overcome these barriers and progressively expand testing from the current one million to three million and then to 30 million tests per week through three action steps.
ACTION STEPS:

To drive rapid scale-up of Covid-19 testing, the ENCT will engage with: producers of testing equipment, reagents, and other lab consumables; national, state and local purchasers; public and private healthcare funders; and financial institutions. The ENCT will also work to identify and resolve choke points in the test supply chain. The ENCT should convene a consensus group of national, state, business, and academic leaders on the use of testing for workplace monitoring and early detection of Covid recurrences. An overarching analysis of the testing supply chain both in the United States and globally should be undertaken immediately.
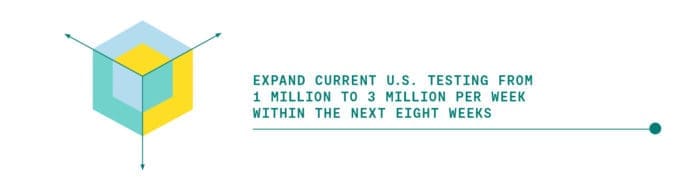
This will be achieved by unleashing the untapped potential of existing test capacity at national, university, and local labs. Importantly, this program would bolster the capacities and resources of thousands of small laboratories around the country. Supply constraints will be identified and eliminated.

This increase will depend on realizing and rolling out the best mix of new technologies for higher efficiency laboratory testing, point-of-care office testing, and home-testing. In addition, some of this increase can be achieved through process efficiencies and lab techniques such as batch sampling. The powers of the Defense Production Act may will be need to be invoked given the inherent commercial uncertainties in this 10-fold production increase.
Launch a Covid Community Healthcare Corps for testing and contact tracing
The taking and preparation of samples, analysis of testing, and human-centered privacy-protected contact tracing will require a massive amount of manpower that can be stood up in the next few weeks by federal, state, and local hiring authorities with funding offered via block grants to states.
The number of tests needed to successfully prevent recurrent outbreaks while allowing some relaxation of social distancing will depend on the vigilance of contact tracing. With the kind of high-precision contact tracing used in South Korea, just 2.5 to 5 million tests per day would be required. With the imprecise tracing of a country like Taiwan, 30 million tests per day would be needed – a level far beyond present capacities.
ACTION STEPS:
 At least 100,000 people and perhaps as many as 300,000 must be hired to undertake a vigorous campaign of test administration and contact tracing, and they must be supported by computer systems networked with regional and national viral datasets and as many electronic health records from local hospital systems as can be provided. The CCHC should designate staff to distribute, administer and oversee testing.
At least 100,000 people and perhaps as many as 300,000 must be hired to undertake a vigorous campaign of test administration and contact tracing, and they must be supported by computer systems networked with regional and national viral datasets and as many electronic health records from local hospital systems as can be provided. The CCHC should designate staff to distribute, administer and oversee testing.
 Policy makers and the public must find the balance between privacy concerns and infection control to allow the infection status of most Americans to be accessed and validated in a few required settings and many voluntary ones.
Policy makers and the public must find the balance between privacy concerns and infection control to allow the infection status of most Americans to be accessed and validated in a few required settings and many voluntary ones.

Whenever possible, incentives should be used to nudge the voluntary use of these apps rather than require them.
Create a Covid-19 Data Commons and Digital Platform
Real-time analyses of resource allocations, disease tracing results and patient medical records will enable policy makers and researchers to make best use of available resources to identify the most promising areas for surges in testing volumes to snuff out Covid-19 recurrent outbreaks and identify the most promising therapeutic treatments and algorithms.
ACTION STEPS:

This effort would support recent Department of Health and Human Services Federal and State collaboration with leading edge data technical firms to develop an integrated, real-time data platform so testing levels can be aligned at regional levels with illness burden. This platform can enhance procurement, distribution and deployment of tests as those tests evolve in quantity and function. It should also enable state and local authorities to track testing results and capacities to identify spot shortages. This will help identify any supply and demand constraints so that testing levels can be aligned at regional levels with illness burdens.

When integrated into national and state surveillance systems, such innovations may enable the same level of outbreak detection with fewer tests. Promising techniques include anonymous digital tracking of workforces or population-based resting heart-rate and smart thermometer trends; continually updated epidemiological data modeling; and artificial intelligence projections based on clinical and imaging data.

This requires that such data be aggregated and examined, while anonymizing personal identification, to determine optimal treatment paradigms and give leads for structured clinical trials.
The Way Forward
Recent reports from the American Enterprise Institute[i], Center for American Progress[ii], Duke Margolis Center[iii], Harvard University Safra Center for Ethics[iv], and Johns Hopkins University[v] each provide unique, complementary perspectives toward a comprehensive approach for relaxing social distancing and reopening our communities and our economy.
Monitoring the pandemic and adjusting social distancing measures will require launching the largest public health testing program in American history. Successful implementation of a national plan to fast-track Covid-19 testing should allow the country to reopen and respond to recurrent outbreaks. The effort will ultimately grow to billions of dollars per month although innovations in testing technology should eventually drop costs. But with widespread business closures costing the country $350 billion to $400 billion each month, the expense will be worth it. This testing infrastructure is intended to tide the country over until a vaccine or therapy is widely available.
Coordination of such a massive program should be treated as a wartime effort, with a public/private bipartisan Pandemic Testing Board established to assist and serve as a bridge between local, state, and federal officials with the logistical, investment and political challenges this operation will inevitably face. Harvard’s Edmond J. Safra Center for Ethics has done an excellent job of outlining possible options (Appendix A). We recommend a combination of federal and state appointed members who would actively serve throughout the crisis.