An Anti-body Test vs. Antigen Test vs. the Lateral Assay Test
Tests for Covid-19 can be divided into polymerise chain reaction (PCR), serologic tests and soon lateral Assay tests. These tests use different kinds of samples to search for different hallmarks of the SARS-CoV-2 virus. PCR (polymerise chain reaction). We take a look at these different approaches and their relative merits.
PCR Molecular Virus Test.
The PCR test detects if the virus is there and someone is actively infected. genetic information of the virus, the RNA. By detecting viral RNA, which will be present in the body before antibodies form or symptoms of the disease are present, the tests can tell whether or not someone has the virus very early on.
PCR tests are used to detect the presence of an antigen, rather than the presence of the body’s immune response, or antibodies.
It’s worth noting that PCR tests can be very labor intensive, with several stages at which errors may occur between sampling and analysis. False negatives can occur up to 30% of the time thus useful for confirming the presence of an infection than giving a patient the all-clear.
This has improved, and with greater automation to reduce errors now have an 80-85% specificity – i.e. the chance the test is detecting the virus.
Serologic testing. Antibody Test.
An antibody test tells you who’s been infected and who should be immune to the virus because the antibodies are generated after a week or two, after which time the virus should have been cleared from the system.
Unlike PCR tests, which commonly use swabs to detect Covid-19, blood samples are usually used for antibody tests. This is because there will be a very small amount of the coronavirus circulating in the blood compared to the respiratory tract, but a significant and measurable antibody presence.
Lateral Flow Assay Test.
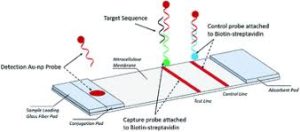
Lateral flow assays have a wide array of applications and can test a variety of samples like urine, blood, saliva, sweat, serum, and other fluids. This new approach for rapid screening test for Covid-19 is a quick-response lateral flow assay that expects produce results in five to 15 minutes, cost under $50 and be administrable by untrained individuals.
All lateral flow tests are designed to identify the presence of a specific biological marker. Pregnancy tests, for example, look for the hormone hCG produced by pregnant people while HIV lateral flow assays detect the virus directly.
In lateral flow tests, particles are used to bind to biological materials and carry them along a test strip, producing a positive or negative result.
technology should make it suitable for in-home testing and monitoring, to help identify if patients need treatment in a clinical facility. It should also be able to verify if people are ready for release from quarantine and to screen individuals prior to entering closed public venues like aeroplanes. This test may be ready by end of April. Several companies are now pursuing approval for such tests.
Rapid in-clinic antigen testing
Bosch has also taken an innovative approach to Covid-19, developing a point of care swab test designed to produce results in under two and a half hours.
Running on Bosch’s pre-existing Vivalytic analysis device, the company says the test is one of the world’s first fully automated molecular diagnostic tests that can be used directly by all medical institutions.
Vivalytic consists of an analyzer device and matching test cartridges. There are biological components in each of the cartridges that are used to prove whether a sample contains SARS-CoV-2 or nine other respiratory viruses. This eliminates the need for further tests if a patient doesn’t have Covid-19, but is presenting with one of the nine other infections and will be made available in Germany in April, with other European markets to follow.
Bosch claims the Covid-19 quick antigen test one of the world’s first fully automated molecular diagnostic tests that is able to determine an infection with SARS-CoV-2 and nine other respiratory viruses within 2.5 hours.
Antibody tests will still be vital in determining immunity that develops among the population, antigen tests can be used to confirm cases of active SARS-CoV-2 infection without PCR’s arduous process of laboratory testing.
BRJ
for the full story: https://www.pharmaceutical-technology.com/features/types-of-covid-19-test-antibody-pcr-antigen/
Keywords: TESTING, PCR, ANTIBODY, SERATOLOGIC TESTS, LATERAL FLOW ASSAY
